FOR US HEALTHCARE PROFESSIONALS ONLY
INVEGA TRINZA® metabolic data observed
Weight change
Change in body weight from the long-term maintenance trial1
| Open-Label Phase (relative to open-label baseline) | Double-Blind Phase (relative to double-blind baseline) | ||
|---|---|---|---|
| Paliperidone Palmitate1* (n=466) | Placebo (n=142) | INVEGA TRINZA® (n=157) | |
| Weight change from baseline | 1.42 kg | -1.28 kg | 0.94 kg |
*During the open-label phase, patients received several doses of INVEGA SUSTENNA® (paliperidone palmitate) followed by a single dose of INVEGA TRINZA®.
WEIGHT GAIN: Weight gain has been reported with atypical antipsychotic use. Clinical monitoring of weight is recommended.1
Fasting glucose
Change in fasting glucose from the long-term maintenance trial1
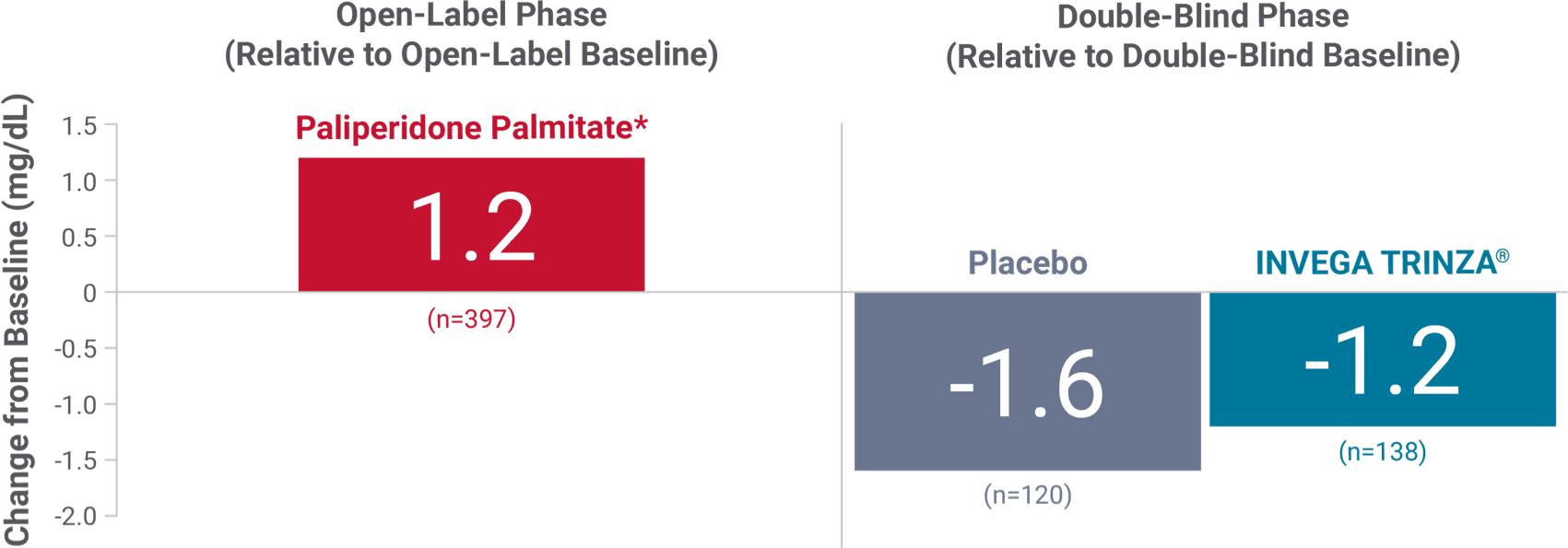
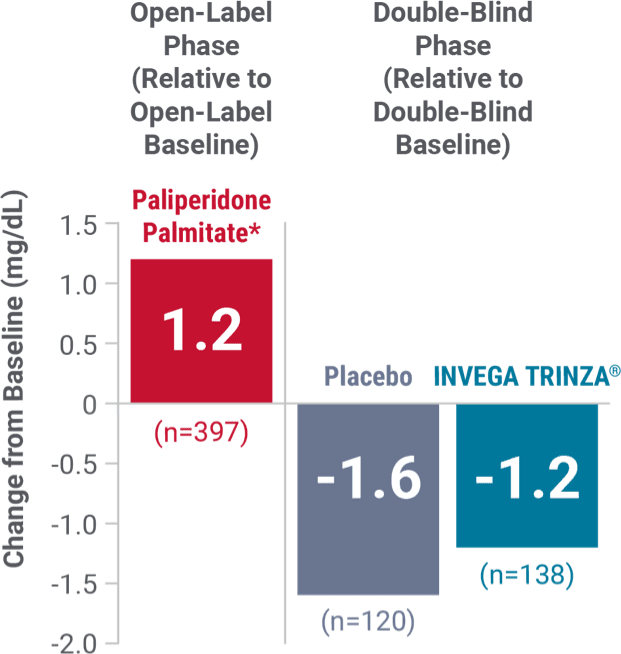
*During the open-label phase, patients received several doses of INVEGA SUSTENNA® followed by a single dose of INVEGA TRINZA®.
Hyperglycemia and diabetes mellitus1
- Monitor for symptoms of hyperglycemia, including polydipsia, polyuria, polyphagia, and weakness
- Monitor glucose regularly in patients with diabetes or at risk for diabetes
Fasting lipids
Change in fasting lipids from the long-term maintenance trial1
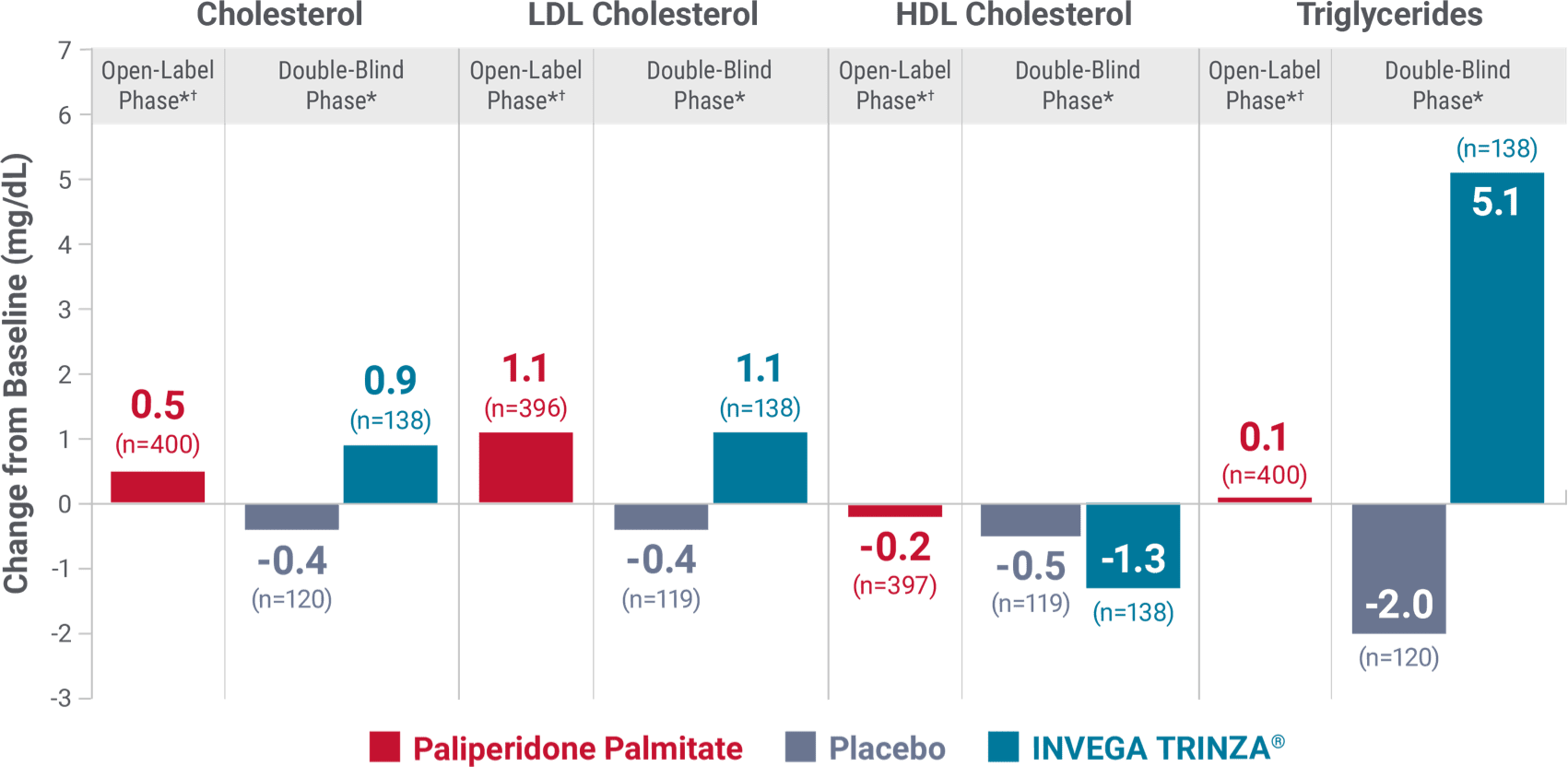
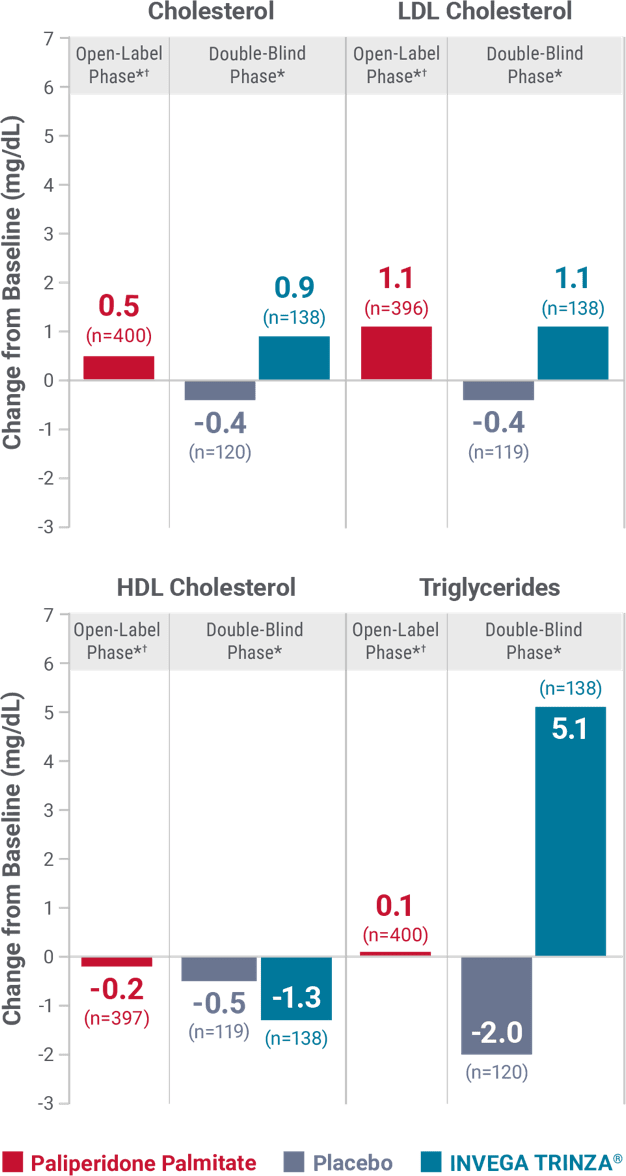
DYSLIPIDEMIA: Undesirable alterations in lipids have been observed in patients treated with antipsychotics.1
*Values for the open-label phase and the double-blind phase are relative to their respective baselines.
†During the open-label phase, patients received several doses of INVEGA SUSTENNA® followed by a single dose of INVEGA TRINZA®.
Reference: 1. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.
Back to Top