FOR US HEALTHCARE PROFESSIONALS ONLY
A noninferiority study compared INVEGA TRINZA® to INVEGA SUSTENNA®
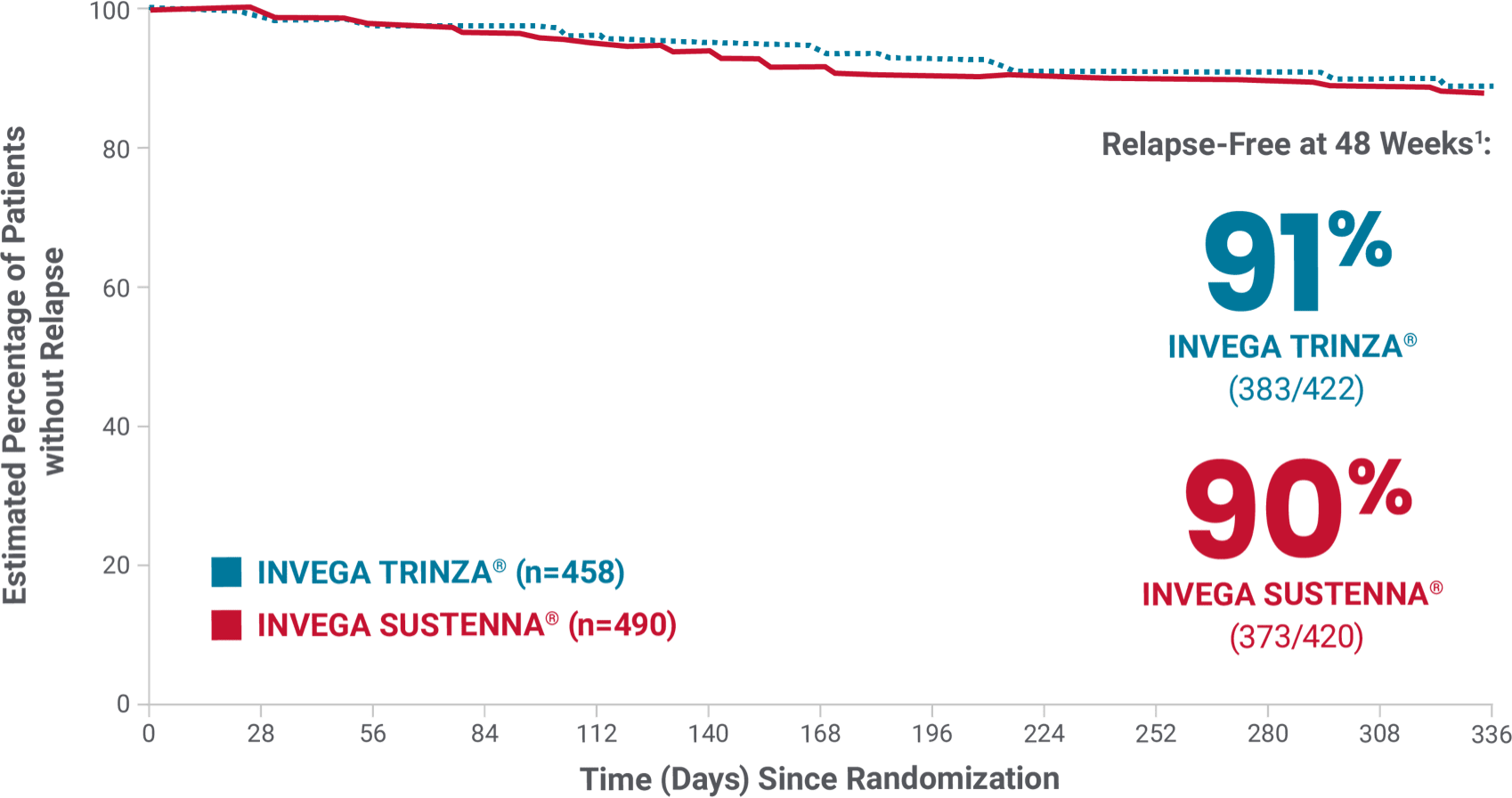
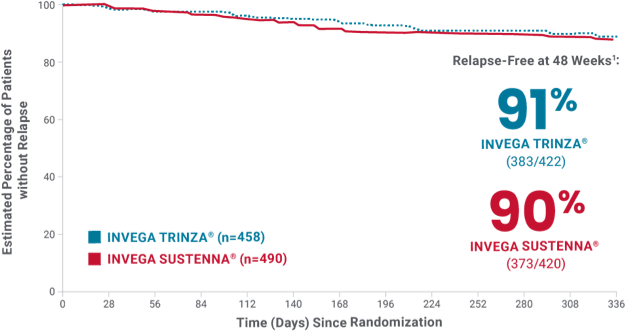
In the study, INVEGA TRINZA® delayed relapse as effectively as INVEGA SUSTENNA®1
Primary endpoint
At Week 48*
91%
of patients taking
INVEGA TRINZA®
VS
90%
of patients taking
INVEGA SUSTENNA®
remained relapse-free1
Secondary endpoint
Mean PANSS total scores were similar for INVEGA SUSTENNA® and INVEGA TRINZA® over time during the double-blind phase1
Nearly 60% of patients in both groups demonstrated symptomatic remission† during the last 6 months of the double-blind phase1‡
- During the double-blind phase, 3% of patients in each group discontinued treatment due to adverse events.
- The most common treatment-emergent adverse events (≥5%) in either group during the double-blind phase were increased weight (21% in both groups), nasopharyngitis (INVEGA TRINZA®: 7%; INVEGA SUSTENNA®: 6%), anxiety (5% in both groups), and headache (INVEGA TRINZA®: 4%; INVEGA SUSTENNA®: 5%)
PANSS=Positive and Negative Syndrome Scale.
*Results of a 48-week, randomized, double-blind noninferiority trial. The prespecified noninferiority margin was -15%. After screening (< 3 weeks) and a 17-week, open-label phase of treatment with INVEGA SUSTENNA®, clinically stable patients were randomized (1:1) to treatment with INVEGA SUSTENNA® or INVEGA TRINZA® for a 48-week double-blind phase. Based on changes from double-blind baseline.
†With one excursion allowed.
‡Symptomatic remission was defined by the Andreasen remission criteria.1 These criteria were developed by an expert working group who reviewed available definitions and assessment instruments to provide a conceptual framework for symptomatic, functional, and cognitive domains in schizophrenia as they relate to remission of illness. The Andreasen criteria are taken from individual scores from all 3 domains of the PANSS, in order to provide a global definition of remission.2
INVEGA TRINZA® was noninferior to INVEGA SUSTENNA® after the 48-week double-blind phase1
Primary endpoint: time to first relapse
- During the double-blind phase, 3% of patients in each group discontinued treatment due to adverse events1
- The most common treatment-emergent adverse events (≥5%) in either group during the double-blind phase were increased weight (21% in both groups), nasopharyngitis (INVEGA TRINZA®: 7%; INVEGA SUSTENNA®: 6%), anxiety (5% in both groups), and headache (INVEGA TRINZA®: 4%; INVEGA SUSTENNA®: 5%)
Relapse criteria
Occurrence of 1 or more of the following:
- Hospitalization for schizophrenia symptoms (involuntary or voluntary admission)
- 25% increase in PANSS total score from randomization on 2 consecutive assessments between 3 and 7 days apart for patients scoring >40 at randomization, or a 10-point increase of patients scoring ≤40 at randomization
- Increase in distinct PANSS item scores (P1, P2, P3, P6, P7, or G8) for 2 consecutive assessments between 3 and 7 days apart
- Clinically significant, deliberate self-injury or violent behavior resulting in suicide, injury, or significant damage
- Suicidal or homicidal ideation and aggressive behavior
PANSS=Positive and Negative Syndrome Scale.
INVEGA TRINZA® achieved similar improvements as INVEGA SUSTENNA® in mean PANSS total scores in the double-blind phase1
Secondary endpoint: change in mean PANSS total score over time
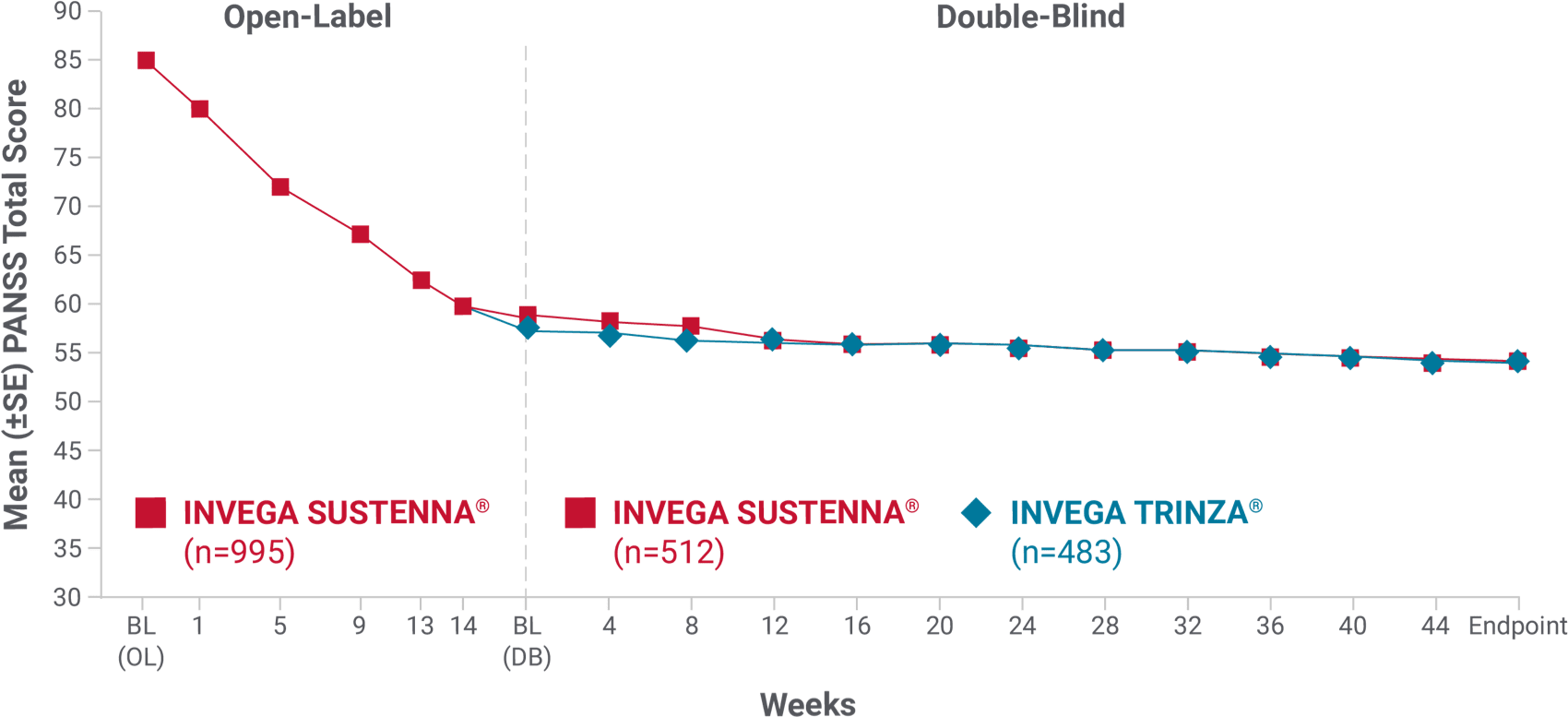
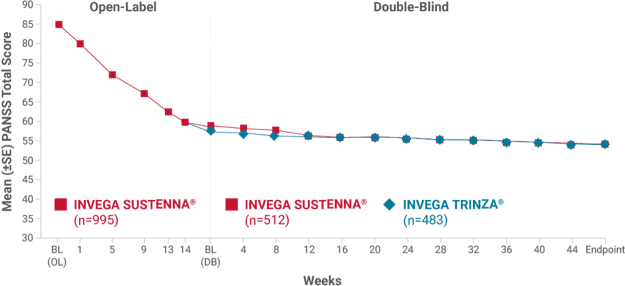
- Double-blind phase: Only patients who were clinically stable* entered the double-blind phase—and they still reported continued improvements in both treatment groups
- The mean baseline PANSS total score for patients entering the double-blind phase in the INVEGA SUSTENNA® group was 58.1, and these patients saw an improvement of -4.3. Patients entering the double-blind phase in the INVEGA TRINZA® group had a mean baseline score of 57.4 and saw an improvement of -3.5
PANSS=Positive and Negative Syndrome Scale.
*Defined as PANSS total score < 70, PANSS item [P1, P2, P3, P6, P7, G8, G14] scores ≤4, and reduction in Clinical Global Impression-Severity (CGI-S) score by ≥1 from open-label baseline.
Patients showed symptomatic remission during the last 6 months of the double-blind phase1

Nearly 6 out of 10 patients in both the INVEGA TRINZA® and INVEGA SUSTENNA® arms showed symptomatic remission*
- 59% of patients taking INVEGA SUSTENNA® and 58% of patients taking INVEGA TRINZA® showed symptomatic remission for the last 6 months of the double-blind phase
- Symptomatic remission was defined by the Andreasen remission criteria
*With one excursion allowed.
Study design: 48-week, randomized, double-blind noninferiority trial1
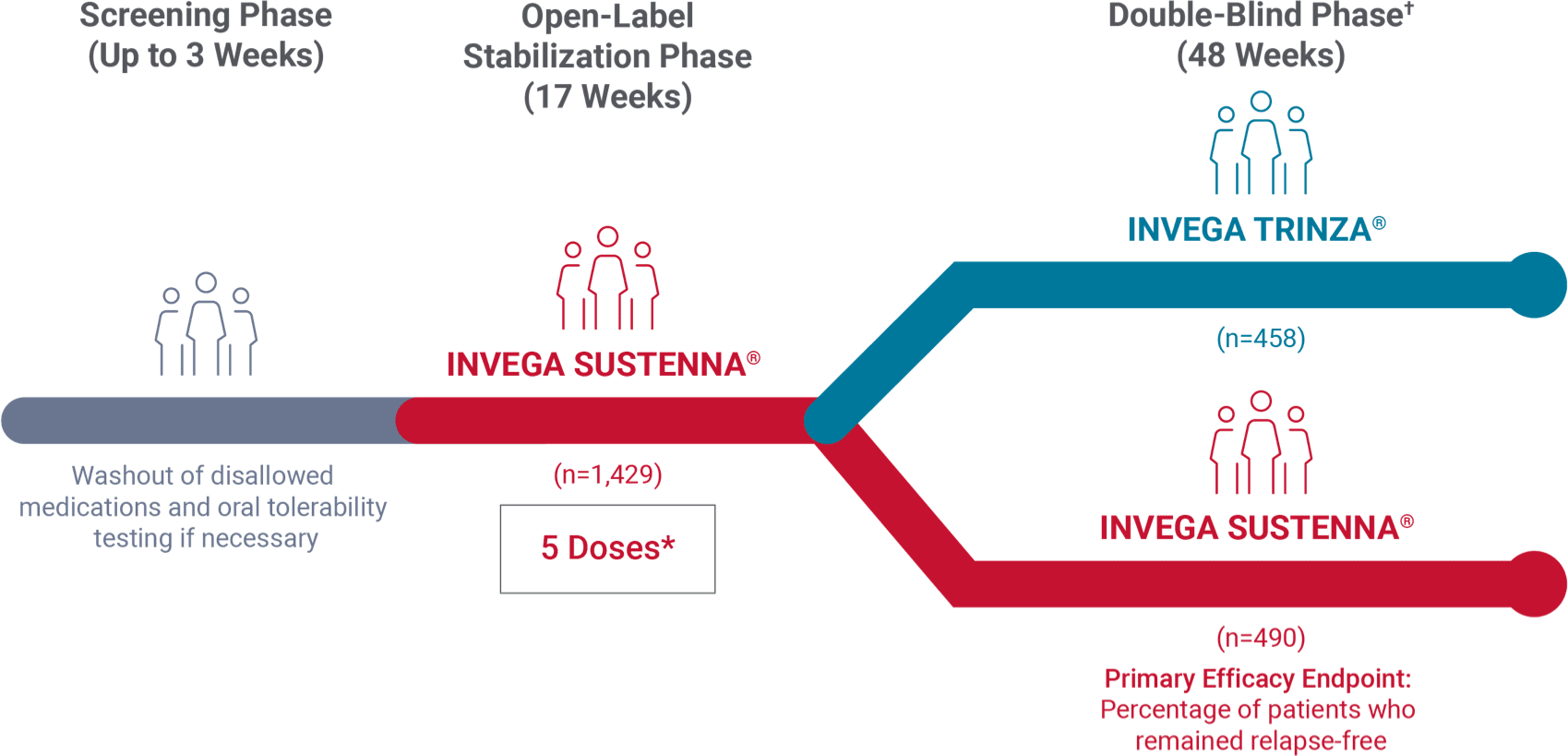
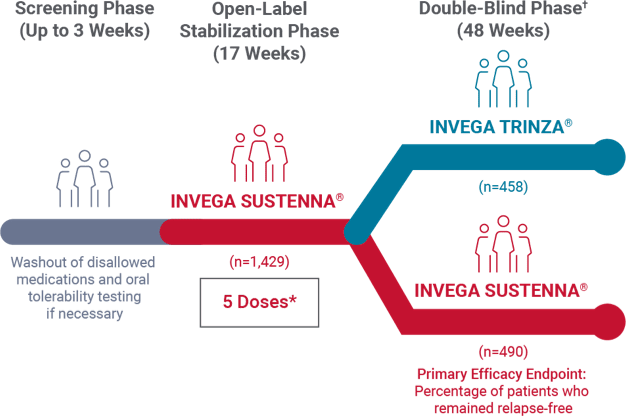
- The prespecified noninferiority margin was -15%
- After a 3-week screening period for oral tolerability testing/washout, patients entered a 17-week open-label stabilization phase (5 doses of INVEGA SUSTENNA®). Clinically stable patients (n=1,016) were then randomized in the double-blind phase to continue receiving INVEGA SUSTENNA® or start on INVEGA TRINZA®
*The 5 doses included the 2 initiation doses and 3 maintenance doses.
†Patients successfully stabilized on INVEGA SUSTENNA® were randomized to continue receiving INVEGA SUSTENNA® or to transition to INVEGA TRINZA®.
Study criteria
Key Inclusion Criteria
- Adult patients (men and women, ages 18-70 years) with a diagnosis of schizophrenia (according to the DSM-IV®), a Positive and Negative Syndrome Scale (PANSS) total score between 70 and 120 at screening and baseline, and worsening of symptoms
- Patients who discontinued other antipsychotics due to insufficient efficacy, safety, or tolerability issues with current therapy, or with preferences for injectable medications
- Women included in the study were postmenopausal, surgically sterile, or used adequate contraception, and eligible men used adequate contraception
Key exclusion Criteria
- Active DSM-IV® diagnosis other than schizophrenia
- Significant risk of suicidal behavior
- History of substance dependence within 6 months before screening
- Involuntary status in a psychiatric hospital at screening
- History of neuroleptic malignant syndrome, tardive dyskinesia, or any unstable or significant medical or neurological illness
- Morbid obesity (BMI >40 kg/m2)
- Other systemic disease, mental retardation, risk factors for prolonged QT interval, torsades de pointes, or sudden death
- History of intolerability, hypersensitivity, or lack of response to risperidone or paliperidone
- Taking any long-acting injectable antipsychotics within 4 weeks before screening
DSM-IV=Diagnostic and Statistical Manual of Mental Disorders, 4th ed.
Back to Top