FOR US HEALTHCARE PROFESSIONALS ONLY
Transition patients to a longer dosing interval with INVEGA TRINZA®1
Once your patient has been adequately treated with a 1-month formulation, you can transition them to a 3-month or 6-month formulation featuring the same active ingredient.
- INVEGA TRINZA® should be administered once every 3 months1
- INVEGA TRINZA® is to be used only after the patient has been adequately treated with 1-month INVEGA SUSTENNA® for at least 4 months1*
- To establish a consistent maintenance dose, it is recommended that the last 2 doses of INVEGA SUSTENNA® be the same dosage strength before starting INVEGA TRINZA®1
- For those who have not taken oral paliperidone, oral risperidone, or injectable risperidone previously, establish tolerability with oral paliperidone or oral risperidone before starting INVEGA SUSTENNA®2
*Based on your evaluation of the patient’s response.
INVEGA TRINZA® initiation doses1
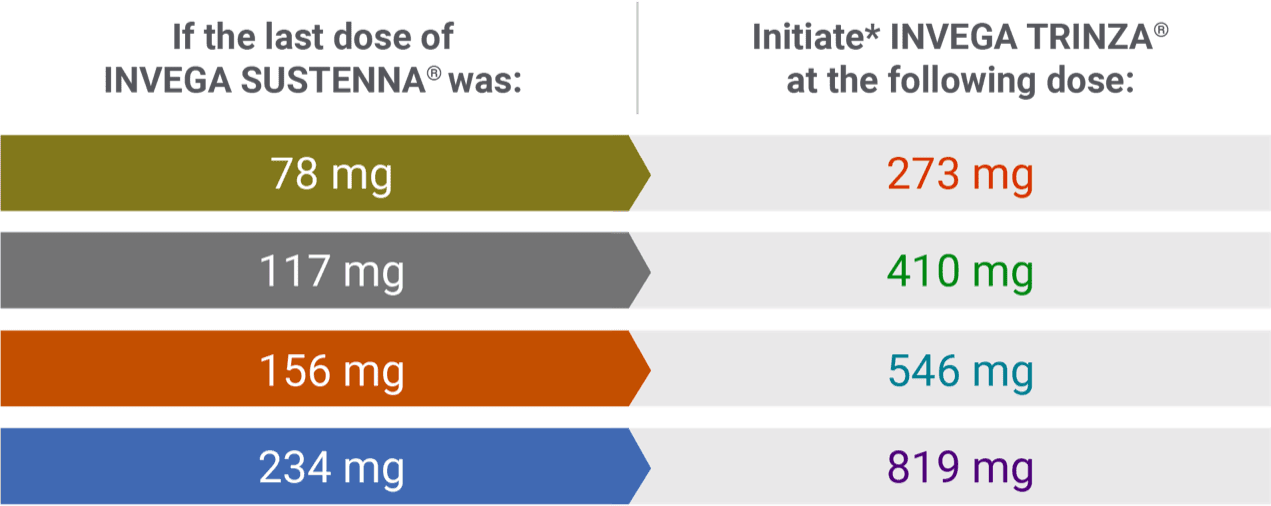
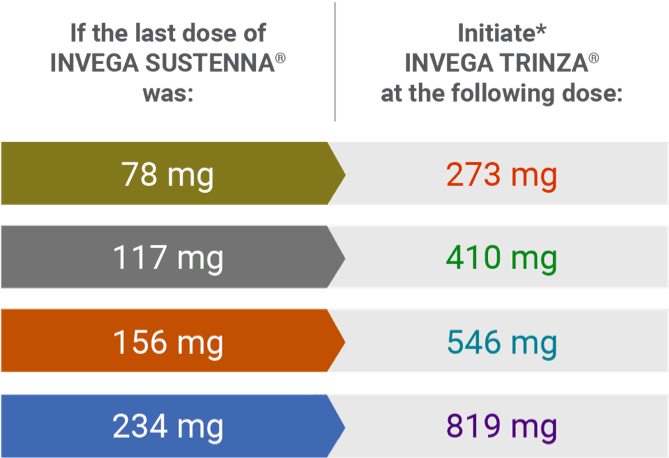
Conversion from the INVEGA SUSTENNA® 39 mg dose was not studied.
After transitioning to INVEGA TRINZA®
Following the initial INVEGA TRINZA® dose, INVEGA TRINZA® should be administered once every 3 months.1
If needed, dose adjustments can be made every 3 months in increments within the range of 273 mg to 819 mg based on tolerability or efficacy.1
Due to the long-acting nature of INVEGA TRINZA®, the patient’s response to an adjusted dose may not be apparent for several months.1
Between doses, patients can maintain scheduled treatment plans and routine interactions with their treatment team.
References: 1. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc. 2. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.
Back to Top
