FOR US HEALTHCARE PROFESSIONALS ONLY
How to administer an INVEGA TRINZA® injection
Instructions for Use Video
Instructions for Use
This video will guide you through the appropriate steps to ensure proper administration of INVEGA TRINZA® three-month paliperidone palmitate. Preparing INVEGA TRINZA® for injection is not the same as preparing INVEGA SUSTENNA® one-month paliperidone palmitate or INVEGA HAFYERA® six-month paliperidone palmitate.
Proper dose preparation is required for complete resuspension of the medication and to reduce the risk of an incomplete injection that could lead to patients not experiencing a full therapeutic response. Please watch this video carefully.
INDICATION
INVEGA HAFYERA®, an every-six-month injection, is an atypical antipsychotic indicated for the treatment of schizophrenia in adults after they have been adequately treated with:
- A once-a-month paliperidone palmitate extended release injectable suspension (e.g., INVEGA SUSTENNA®) for at least four months or
- An every-three-month paliperidone palmitate extended release injectable suspension (e.g., INVEGA TRINZA®) for at least one three-month cycle.
INVEGA TRINZA® is an atypical antipsychotic indicated for the treatment of schizophrenia in patients after they have been adequately treated with INVEGA SUSTENNA® for at least four months.
INVEGA SUSTENNA® is an atypical antipsychotic indicated for the treatment of schizophrenia in adults.
IMPORTANT SAFETY INFORMATION.
Warning, increased mortality in elderly patients with dementia-related psychosis. See full Prescribing Information for complete Boxed Warning. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. INVEGA HAFYERA®, INVEGA TRINZA® and INVEGA SUSTENNA® are not approved for use in patients with dementia-related psychosis.
- INVEGA TRINZA® should be administered by a healthcare professional as a single injection
- Do not divide dose into multiple injections
- INVEGA TRINZA® is intended for intramuscular use only
- Inject slowly, deep into the muscle taking care to avoid injection into a blood vessel
- Please read complete instructions prior to use
Step one, selecting the needle.
INVEGA TRINZA® has components and steps for preparation that are unique. Let's start by going through all the contents of the dose pack. Each dose pack of INVEGA TRINZA® should include a prefilled syringe and two thin wall safety needles. Thin wall safety needles are designed to be used with INVEGA TRINZA®. Therefore, it is important to only use the needles provided in the INVEGA TRINZA® kit. First, select the proper needle based on the injection area and the weight of your patient. If administering a deltoid injection and your patient weighs less than 200 pounds or 90 kilograms, choose the 22-gauge one-inch needle with the pink hub.
If your patient weighs 200 pounds or more, or 90 kilograms or more, choose the 22-gauge one-and-a-half-inch needle with the yellow hub. For all gluteal injections, however, no needle choice is necessary. Only use the 22-gauge one-and-a-half-inch needle with the yellow hub, regardless of patient weight.
INVEGA TRINZA® is injected into the upper-outer quadrant of the gluteal muscle. There is no need for the patient to fully disrobe.
We've now reached one of the most important steps in preparing INVEGA TRINZA®. Please note: INVEGA TRINZA® requires longer and more vigorous shaking than INVEGA SUSTENNA® because it is a more concentrated suspension. Proper dose preparation is required for complete resuspension of the medication and to reduce the risk of an incomplete injection that could lead to patients not experiencing a full therapeutic response. INVEGA TRINZA® must be shaken vigorously, with the syringe tip pointing up, for at least 15 seconds. Proper suspension prior to injection is absolutely essential to successful delivery, so keep your eye on the timer and follow this technique to make sure you're shaking INVEGA TRINZA® the right way.
With the syringe tip pointing up, ensure that the prefilled syringe is shaken vigorously for at least 15 seconds within 5 minutes prior to administration to ensure a homogeneous suspension and ensure the needle does not get clogged during injection. 13, 14, 15 done. Now, check the liquid in the viewing window of the syringe. The suspension should appear uniform and milky white. It is also normal to see small air bubbles. Now that you've properly shaken the suspension, you have five minutes to inject it. Proceed to the next step immediately after shaking. Remember, if more than 5 minutes pass before injection shake vigorously again for at least 15 seconds with a syringe tip pointing up to re-suspend the medication.
Now you're ready to prepare for administering the injection. Open the needle pouch by peeling the cover back halfway and place it on a clean surface. Then, holding the syringe upright, twist and pull the rubber cap to remove it. Fold back the needle cover and plastic tray. Hold the syringe pointing up. Attach the safety needle to the syringe using a gentle twisting motion to avoid needle hub cracks or damage. Always check for signs of damage or leakage prior to administration. Be sure you do not remove the pouch until the syringe and needle are securely attached. Pull the needle sheath away from the needle in a straight motion. Do not twist the sheath, as this may loosen the needle from the syringe.
Hold the syringe upright and tap gently to make any air bubbles rise to the top. Remove air by pressing the plunger rod upward carefully until a drop of the liquid comes out of the needle tip. You've prepared the dose. Now you're ready to inject. Wipe the injection site with an alcohol swab and allow to dry. Do not touch, fan, or blow on the injection site after you have cleaned it. Slowly inject the entire contents of the syringe intramuscularly deep into the selected deltoid or gluteal muscle. Do not administer by any other route. There may be a small amount of blood or liquid at the injection site. Hold pressure on the skin with a cotton ball or gauze pad until any bleeding stops. Do not rub the injection site. If needed, cover the injection site with a bandage.
After the injection is complete use your thumb or a flat surface to secure the needle in the safety device. The needle is secure when a click sound is heard. Dispose of the syringe and unused needle, if not already done earlier, in an approved sharps container. Thank you for watching this video. Please continue watching to view important safety information, including Boxed Warning for INVEGA TRINZA®.
INDICATION
INVEGA HAFYERA®, an every-six-month injection, is an atypical antipsychotic indicated for the treatment of schizophrenia in adults after they have been adequately treated with:
- A once-a-month paliperidone palmitate extended release injectable suspension (e.g., INVEGA SUSTENNA®) for at least four months or
- An every-three-month paliperidone palmitate extended release injectable suspension (e.g., INVEGA TRINZA®) for at least one three-month cycle.
INVEGA TRINZA® is an atypical antipsychotic indicated for the treatment of schizophrenia in patients after they have been adequately treated with INVEGA SUSTENNA® for at least four months.
INVEGA SUSTENNA® is an atypical antipsychotic indicated for the treatment of schizophrenia in adults.
IMPORTANT SAFETY INFORMATION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS.
See full prescribing information for complete Boxed Warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. INVEGA HAFYERA®, INVEGA TRINZA® and INVEGA SUSTENNA® are not approved for use in patients with dementia-related psychosis.
Contraindications. INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® are contraindicated in patients with a known hypersensitivity to either paliperidone, risperidone, or to any excipients of their formulation.
Cerebrovascular adverse reactions. Cerebrovascular adverse reactions, for example, stroke, transient ischemic attacks, including fatalities, were reported at a higher incidence in elderly patients with dementia-related psychosis taking risperidone, aripiprazole, and olanzapine compared to placebo. No studies have been conducted with oral paliperidone, INVEGA HAFYERA®, INVEGA TRINZA®, or INVEGA SUSTENNA® in elderly patients with dementia. These medications are not approved for the treatment of patients with dementia-related psychosis.
Neuroleptic malignant syndrome (NMS). NMS, a potentially fatal symptom complex, has been reported in association with antipsychotic drugs, including paliperidone. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, including delirium and autonomic instability (irregular pulse of blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine, phosphokinase, myoglobinuria, rhabdomyolysis, and acute renal failure. If NMS is suspected, immediately discontinue INVEGA HAFYERA®, INVEGA TRINZA®, or INVEGA SUSTENNA®, and provide symptomatic treatment and monitoring.
QT prolongation. Paliperidone causes a modest increase in the corrected QT (QTc) interval. Avoid the use of drugs that also increase QTc interval and in patients with risk factors for prolonged QTc interval.
Paliperidone should also be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias. Certain circumstances may increase the risk of the occurrence of torsades de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval.
Tardive dyskinesia (TD). TD, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing TD, and the likelihood that it will become irreversible, appear to increase with the duration of treatment and the cumulative dose. The syndrome can develop after relatively brief treatment periods, even at low doses. It may also occur after discontinuation. TD may remit partially or completely if antipsychotic treatment is discontinued. Antipsychotic treatment itself, however, may suppress or partially suppress the signs and symptoms of the syndrome possibly masking the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
If signs and symptoms of TD appear in a patient on INVEGA HAFYERA®, INVEGA TRINZA®, or INVEGA SUSTENNA®, drug discontinuation should be considered. However, some patients may require treatment with INVEGA HAFYERA®, INVEGA TRINZA®, or INVEGA SUSTENNA® despite the presence of the syndrome. In patients who do require chronic treatment, use the lowest dose and the shortest duration of treatment, producing a satisfactory clinical response. Periodically reassess the need for continued treatment.
Metabolic changes. Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While all of the drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia and diabetes mellitus. Hyperglycemia and diabetes mellitus, in some cases extreme and associated with ketoacidosis, hyperosmolar coma or death have been reported in patients treated with all atypical antipsychotics (APS). Patients starting treatment with APS who have or are at risk for diabetes mellitus should undergo fasting blood glucose testing at the beginning of and during treatment. Patients who develop symptoms of hyperglycemia during treatment should also undergo fasting blood glucose testing. All patients treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia. Some patients require continuation of antidiabetic treatment despite discontinuation of the suspect drug.
Dyslipidemia. Undesirable alterations have been observed in patients treated with atypical antipsychotics.
Weight gain. Weight gain has been observed with atypical antipsychotic use. Clinical monitoring of weight is recommended.
Orthostatic hypotension and syncope. INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® may induce orthostatic hypotension in some patients due to its alpha-adrenergic blocking activity. INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® should be used with caution in patients with known cardiovascular disease, cerebrovascular disease or conditions that would predispose patients to hypotension (for example, dehydration, hypovolemia, treatment with antihypertensive medications). Monitoring should be considered in patients for whom this may be of concern.
Falls. Somnolence, postural hypotension, motor and sensory instability have been reported with the use of antipsychotics, including INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA®, which may lead to falls and consequently fractures or other fall-related injuries. For patients, particularly the elderly with diseases, conditions, or medications that could exacerbate these effects, assess the risk of falls when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
Leukopenia, neutropenia and agranulocytosis have been reported with antipsychotics, including INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA®. In patients with a history of clinically significant low white blood cell count (WBC)/absolute neutrophil count (ANC) or drug-induced leukopenia/neutropenia, perform a complete blood count frequently during the first few months of therapy. Consider discontinuing INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® at the first sign of a clinically significant decline in WBC in the absence of other causative factors. Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection, and treat promptly if such symptoms or signs occur. Discontinue INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® in patients with severe neutropenia (absolute neutrophil count less than 1000/mm3) and follow their WBC until recovery.
Hyperprolactinemia. As with other drugs that antagonize dopamine D2 receptors, INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® elevate prolactin levels, and the elevation persists during chronic administration. Paliperidone has a prolactin-elevating effect similar to risperidone, which is associated with higher levels of prolactin elevation than other antipsychotic agents.
Potential for cognitive and motor impairment. Somnolence, sedation, and dizziness were reported as adverse reactions in subjects treated with INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA®. INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® have the potential to impair judgment, thinking, or motor skills. Patients should be cautioned about performing activities that require mental alertness such as operating hazardous machinery, including motor vehicles, until they are reasonably certain that INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® do not adversely affect them.
Seizures. INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® should be used cautiously in patients with a history of seizures or with conditions that potentially lower seizure threshold. Conditions that lower seizure threshold may be more prevalent in patients 65 years or older.
Administration. for intramuscular injection only by a healthcare professional using only the needles provided in the INVEGA HAFYERA®, INVEGA TRINZA®, or INVEGA SUSTENNA® kits. Care should be taken to avoid inadvertent injection into a blood vessel.
Drug interactions. Strong CYP3A4/P-glycoprotein (P-gp) inducers. Avoid using a strong inducer of CYP3A4 and/or P-gp (for example, carbamazepine, rifampin, St. John's Wort) during a dosing interval for INVEGA HAFYERA®, INVEGA TRINZA®, or INVEGA SUSTENNA®. If administering a strong inducer is necessary, consider managing the patient using paliperidone extended-release tablets.
Pregnancy/Nursing. INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® may cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. Advise patients to notify their healthcare professional if they become pregnant or intend to become pregnant during treatment with INVEGA HAFYERA®, INVEGA TRINZA®, or INVEGA SUSTENNA®. Patients should be advised that there is a pregnancy registry that monitors outcomes in women exposed to INVEGA HAFYERA®, INVEGA TRINZA®, or INVEGA SUSTENNA® during pregnancy. INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA® can pass into human breast milk. The benefits of breastfeeding should be considered along with the mother's clinical need for INVEGA HAFYERA®, INVEGA TRINZA®, or INVEGA SUSTENNA®, and any potential adverse effect on the breastfed infant from INVEGA HAFYERA®, INVEGA TRINZA®, or INVEGA SUSTENNA®, or the mother's underlying condition.
Commonly observed adverse reactions for INVEGA HAFYERA®. The most common adverse reactions (incidence at least 5% in the double-blind phase) in the INVEGA HAFYERA® year clinical trial were upper respiratory tract infection, injection site reaction, weight increased, headache, and parkinsonism.
Commonly observed adverse reactions for INVEGA TRINZA®. The most common adverse reactions (incidence greater than or equal to 5% and occurring at least twice as often as placebo) were injection site reaction, weight increased, headache, upper respiratory tract infection, akathisia, and parkinsonism.
Commonly observed adverse reactions for INVEGA SUSTENNA®. The most common adverse reactions in clinical trials in patients with schizophrenia (incidence greater than or equal to 5% and occurring at least twice as often as placebo) were injection site reactions, somnolence, sedation, dizziness, akathisia, and extrapyramidal disorder.
Please read the full Prescribing Information, including Boxed Warning, for INVEGA HAFYERA®, INVEGA TRINZA®, and INVEGA SUSTENNA®.
INVEGA TRINZA® needle selection and injection site are key to a successful patient experience
INVEGA TRINZA® dose pack contents1
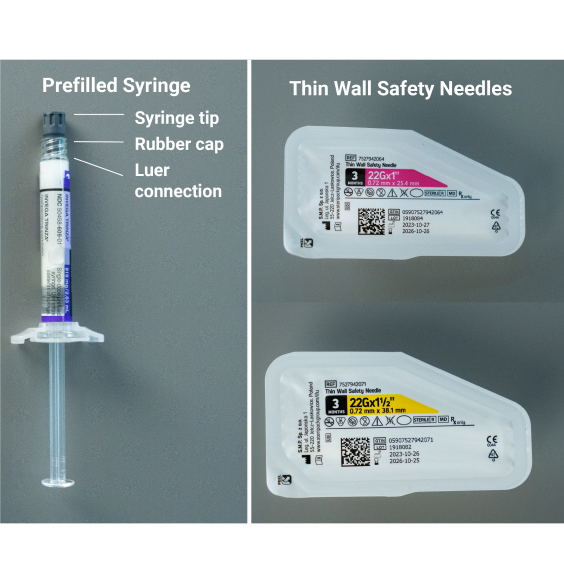
Select needle1
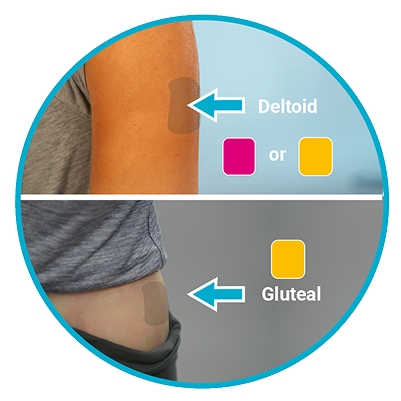
Needle selection is determined by injection area and patient weight.

If administering a deltoid injection
- If patient weighs <90 kg:
Pink Hub | 22G x 1” - If patient weighs >90kg:
Yellow Hub | 22G x 1½”

If administering a gluteal injection
- If patient weighs <90 kg:
Yellow Hub | 22G x 1½” - If patient weighs >90kg:
Yellow Hub | 22G x 1½”
IMPORTANT: Immediately discard the unused needle in an approved sharps container. Do not save for future use.
Preparing and injecting INVEGA TRINZA®1

Step 1
SHAKE VIGOROUSLY for at least 15 seconds1
With the syringe tip pointing up, SHAKE VIGOROUSLY with a loose wrist for at least 15 seconds to ensure a homogeneous suspension.
NOTE: This medication requires longer and more vigorous shaking than the 1-month paliperidone palmitate extended-release injectable suspension.
IMPORTANT: Proceed to the next step immediately after shaking. If more than 5 minutes pass before injection, shake vigorously, with the syringe tip pointing up, again for at least 15 seconds to resuspend the medication.
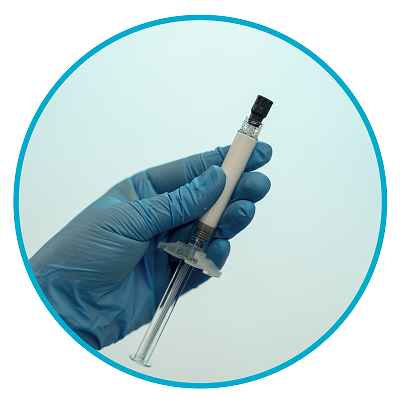
Step 2
Check suspension1
After shaking the syringe for at least 15 seconds, check the liquid in the viewing window. The suspension should appear uniform and milky white in color. It is also normal to see small air bubbles.

Step 3
Open the INVEGA TRINZA® needle pouch and remove cap1
First, open needle pouch by peeling the cover back halfway. Place on a clean surface.
Then, holding the syringe upright, twist and pull the rubber cap to remove.
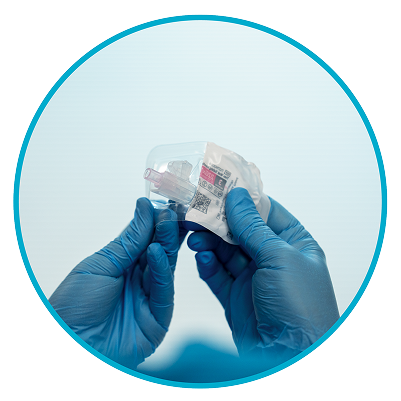
Step 4
Grasp needle pouch1
Fold back needle cover and plastic tray. Then, firmly grasp the needle sheath through the pouch, as shown.

Step 5
Attach needle1
Hold the syringe pointing up. Attach the safety needle to the syringe using a gentle twisting motion to avoid needle hub cracks or damage. Always check for signs of damage or leakage prior to administration.
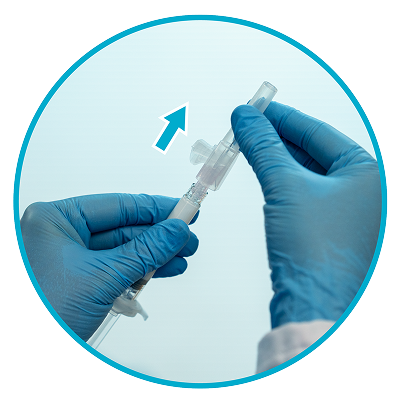
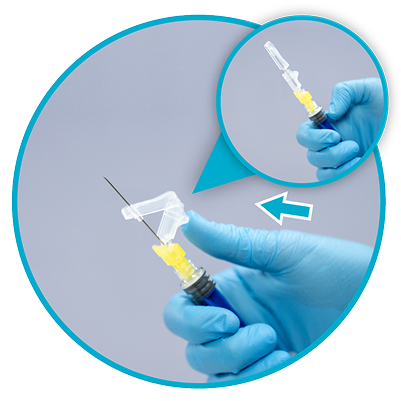
Step 6
Remove needle sheath1
Pull the needle sheath away from the needle in a straight motion.
Do not twist the sheath, as this may loosen the needle from the syringe.
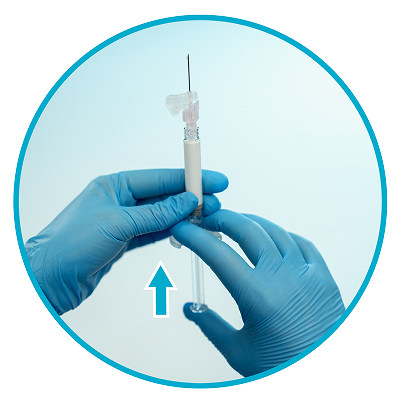
Step 7
Remove air bubbles1
Hold the syringe upright and tap gently to make any air bubbles rise to the top.
Remove air by pressing the plunger rod upward carefully until a drop of liquid comes out of the needle tip.
Step 8
Inject dose1
Slowly inject the entire contents of the syringe intramuscularly, deep into the selected deltoid or the upper-outer quadrant of the gluteal muscle.
Do not administer by any other route.
Step 9
Secure needle1
After the injection is complete, use your thumb or a flat surface to secure the needle in the safety device.
The needle is secure when a “click” sound is heard.
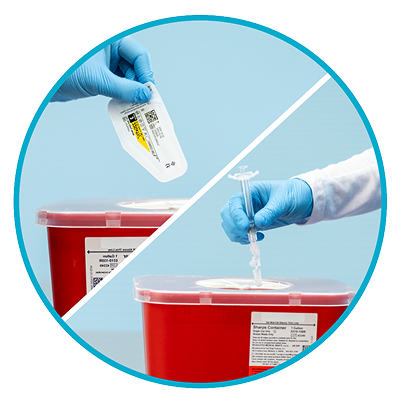
Step 10
Dispose properly1
Dispose of the syringe and unused needle in an approved Sharps Container.
IMPORTANT: Thin wall safety needles are designed specifically for use with INVEGA TRINZA®. Unused needle should be discarded and not saved for future use.
Important administration information1
- INVEGA TRINZA® should be administered by a healthcare professional as a single injection. DO NOT divide dose into multiple injections
- INVEGA TRINZA® is intended for intramuscular use only. Inject slowly, deep into the muscle, taking care to avoid injection into a blood vessel
- Read complete instructions prior to use
Dosing1
This medication should be administered once every 3 months.
Thin wall safety needle selection1
Thin wall safety needles are designed to be used with INVEGA TRINZA®. Therefore, it is important to only use the needles provided in the INVEGA TRINZA® kit.
Preparation1
- Peel off tab label from the syringe and place in patient record
- INVEGA TRINZA® requires longer and more vigorous shaking than INVEGA SUSTENNA® (1-month paliperidone palmitate extended-release injectable suspension). Shake the syringe vigorously with a loose wrist, with the syringe tip pointing up, for at least 15 seconds within 5 minutes prior to administration
Reference: 1. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.
Back to Top
