Before transitioning to INVEGA SUSTENNA®,
establish tolerability in patients who have not taken oral paliperidone, oral risperidone, or injectable risperidone1. Learn about initiation and maintenance dosing for INVEGA SUSTENNA®.
A few simple adjustments in communication and integration between treatment teams may also help your patients make a successful transition between care settings.
Download these actionable best practices to address 7 common challenges associated with the continuity of care.
LAI=long-acting injectable.
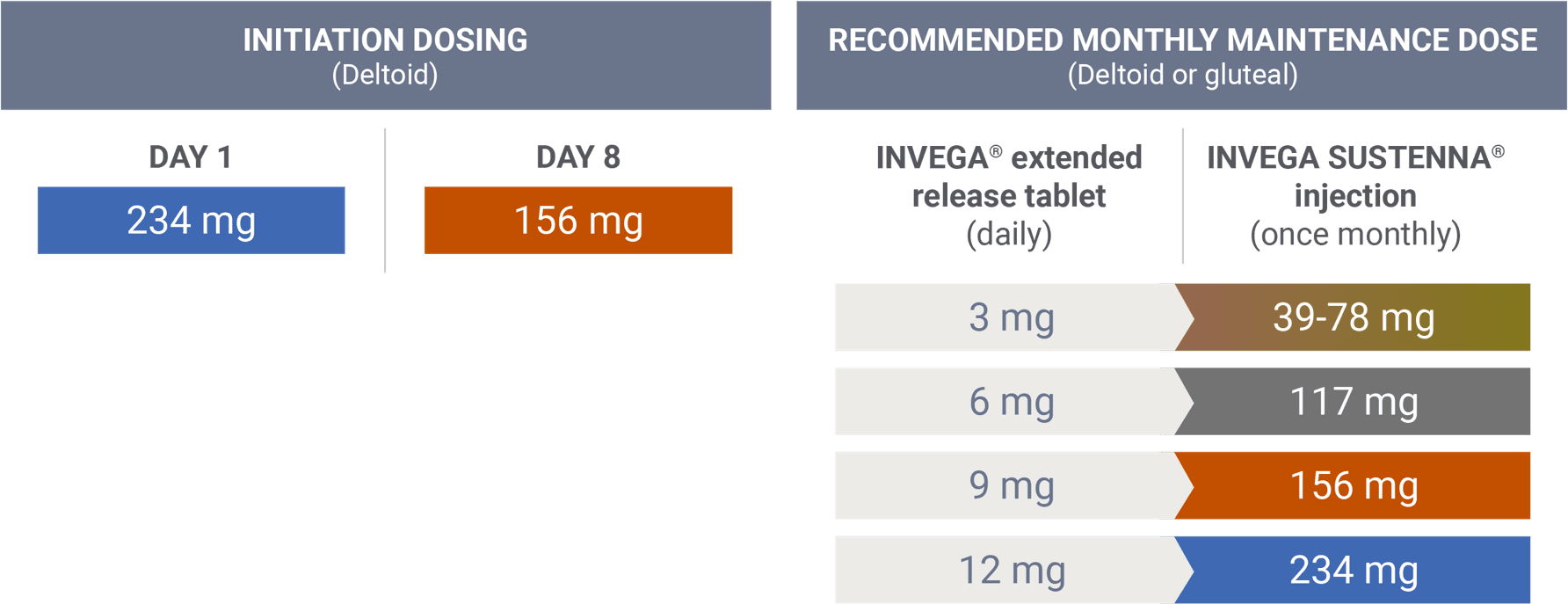
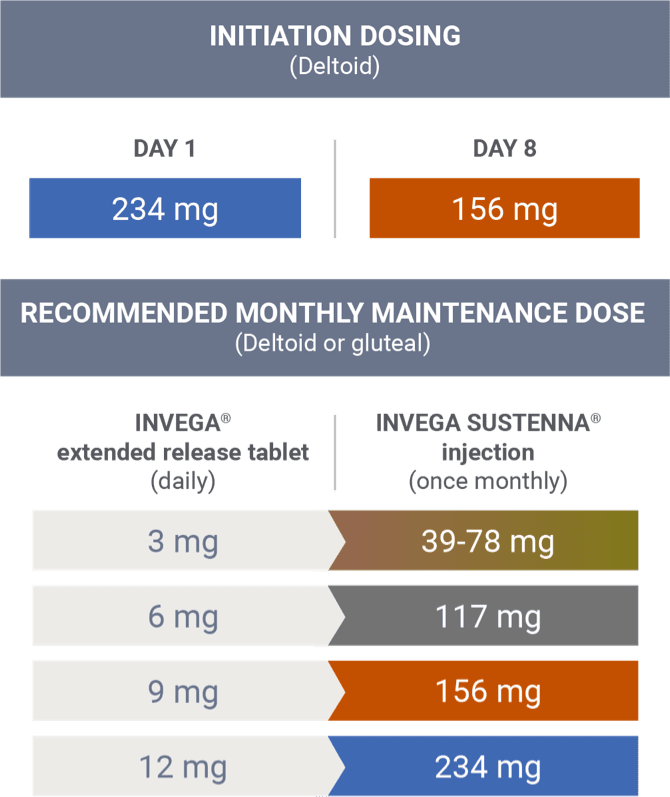
Transitioning from INVEGA® (paliperidone) extended-release tablets
Transitioning from risperidone tablets2
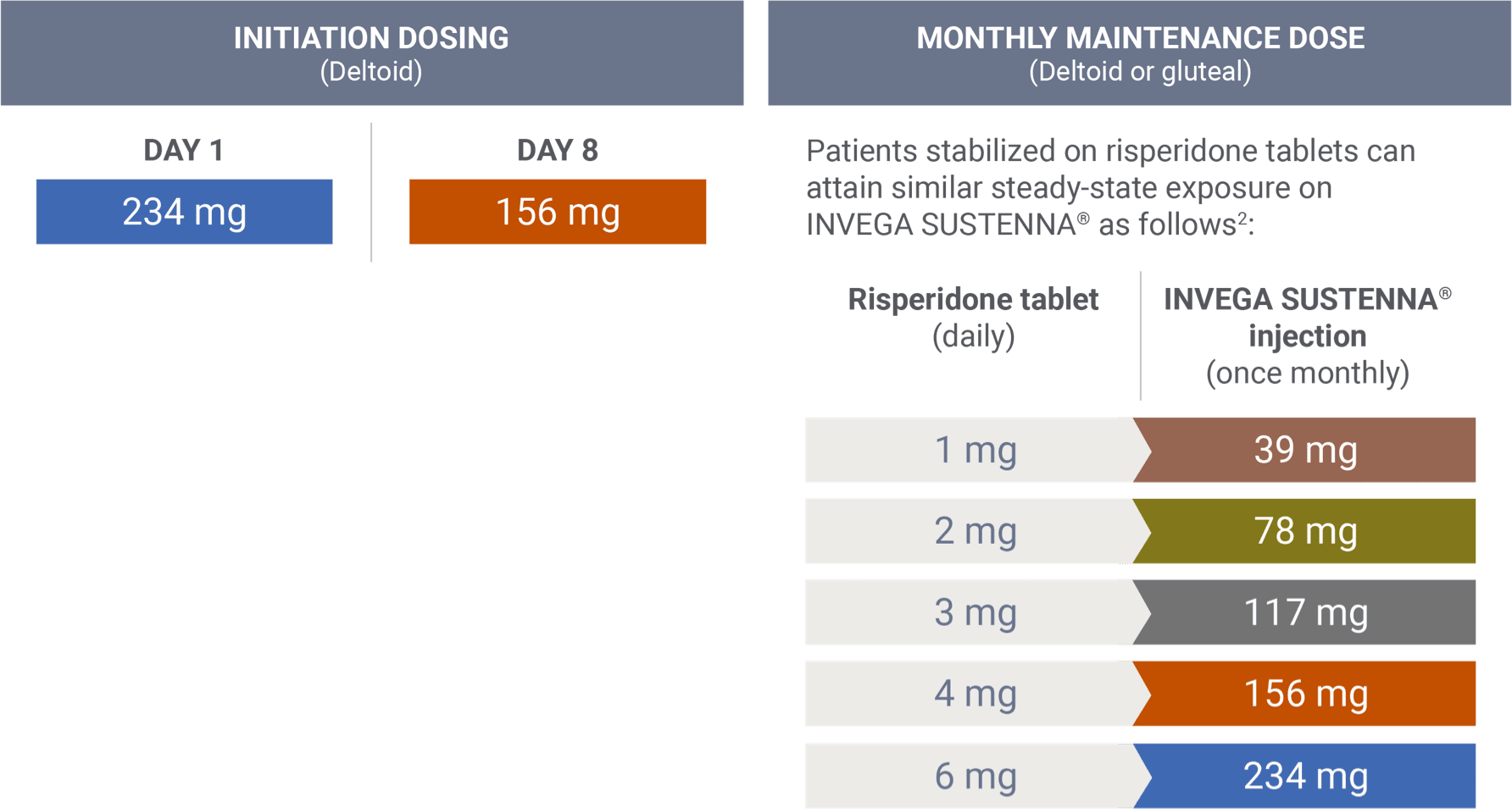
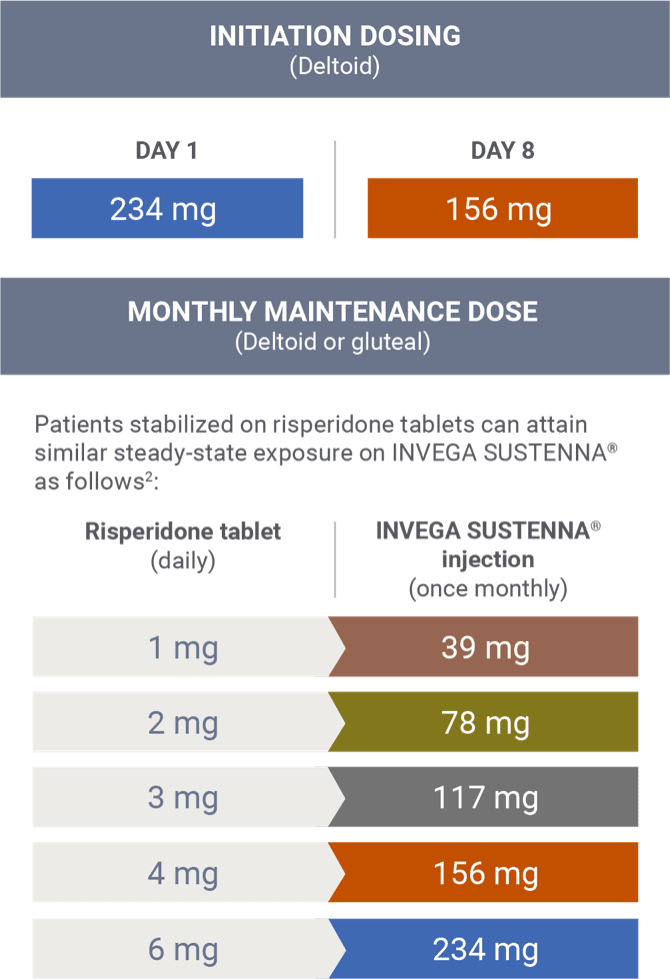
There are no systematically collected data to specifically address transitioning patients with schizophrenia from other antipsychotics to INVEGA SUSTENNA®.1
This information is based on pharmacokinetic (PK) modeling performed to compare steady-state exposure during maintenance treatment between risperidone tablets and INVEGA SUSTENNA® (after both the 234 mg/156 mg deltoid starting doses).2 This information is not included in the INVEGA SUSTENNA® Prescribing Information.
Additional maintenance dosing information1
- The first monthly maintenance dose should be administered 5 weeks after the first injection (regardless of the timing of the second injection)
- The recommended maintenance dose for the treatment of schizophrenia is 117 mg
- Some patients may benefit from lower or higher maintenance doses within the additional available strengths (39 mg, 78 mg, 156 mg, and 234 mg)
Transitioning from RISPERDAL CONSTA® (risperidone)1,3
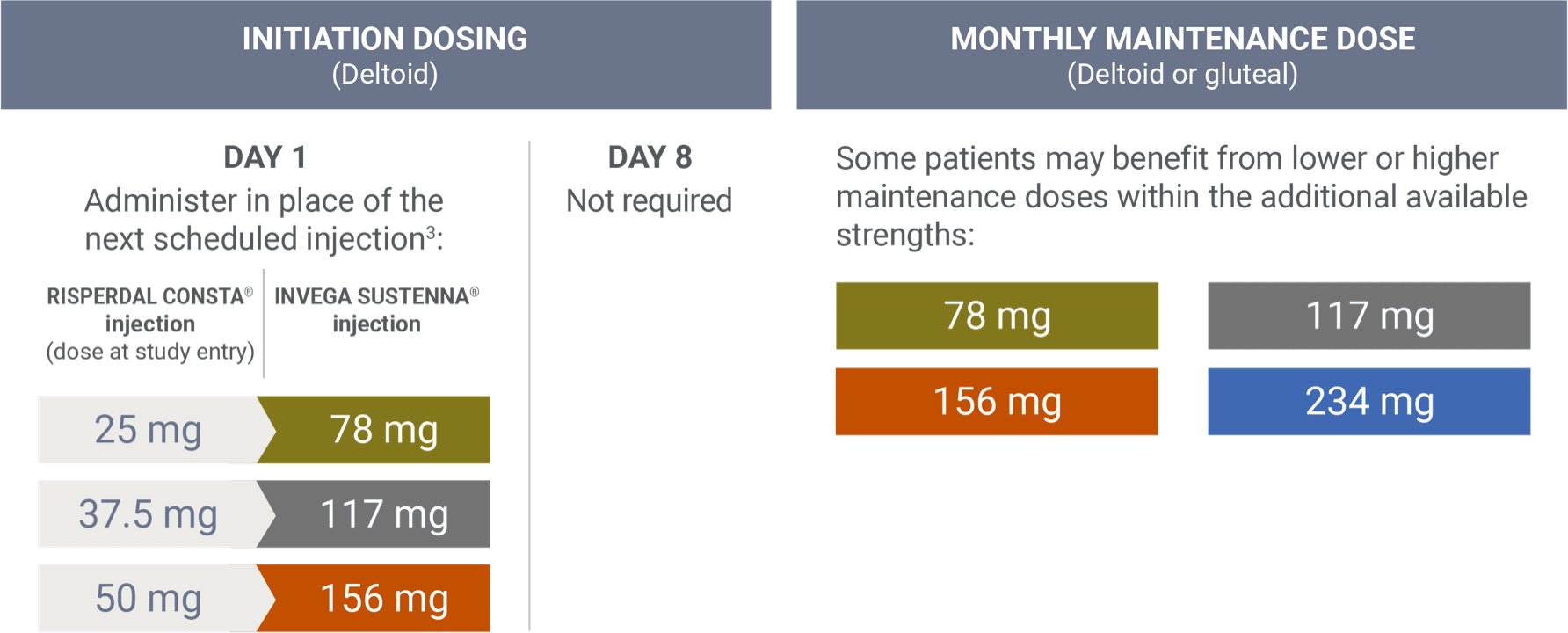
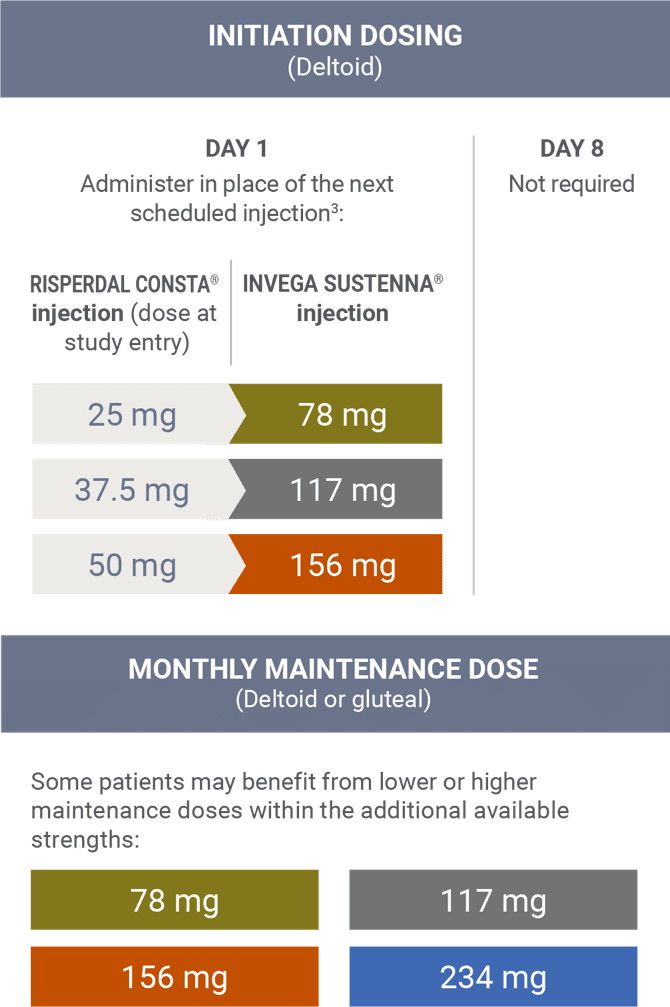
Transition dosing was based on pharmacokinetic modeling. The Prescribing Information for INVEGA SUSTENNA® and RISPERDAL CONSTA® does not include conversion charts between the 2 agents.3
During the open-label stabilization phase of a long-term maintenance trial for INVEGA TRINZA® for the treatment of schizophrenia, enrolled patients treated with RISPERDAL CONSTA® long-acting injection were switched to INVEGA SUSTENNA® in place of the next scheduled injection at a dose determined by the conversion guide.4 The INVEGA SUSTENNA® conversion dose may not reflect the eventual stabilization dose that was achieved during the remainder of the open-label transition phase.
Transitioning from long-acting injectable (LAI) antipsychotics1
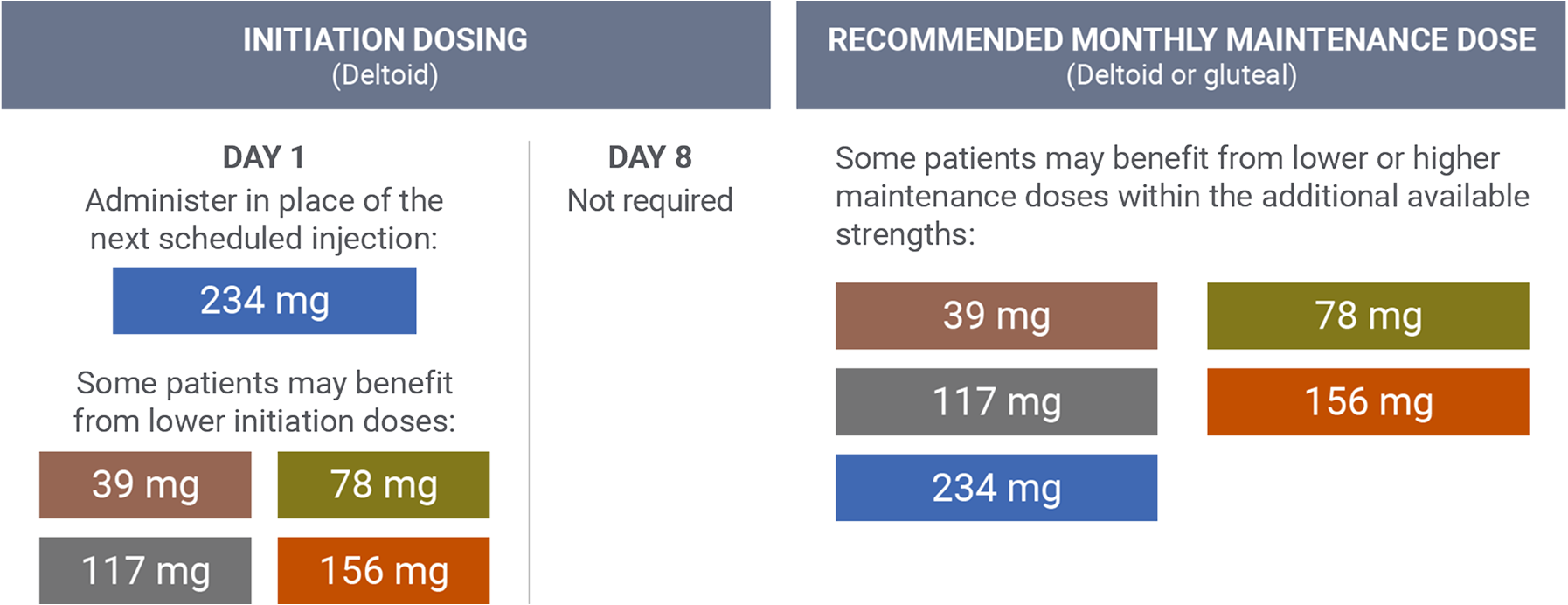
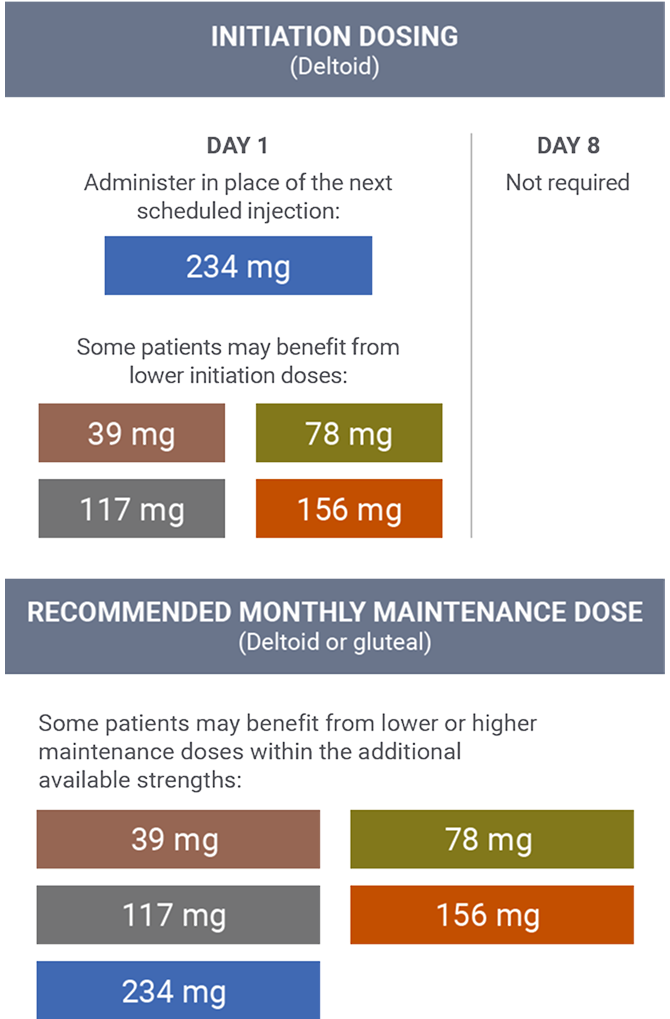
The 234 mg INVEGA SUSTENNA® strength was used in the pivotal clinical trial for INVEGA TRINZA® (paliperidone palmitate) as an initiation dose for patients who were being transitioned from another LAI antipsychotic.
Additional maintenance dosing information1
- Administered 1 month after the initial dose
- The recommended maintenance dose for the treatment of schizophrenia is 117 mg
- Some patients may benefit from lower or higher maintenance doses within the additional available strengths (39 mg, 78 mg, 156 mg, and 234 mg)
- The INVEGA SUSTENNA® conversion dose may not reflect the eventual stabilization dose that was achieved during the remainder of the open-label transition phase
No oral supplementation needed1
For patients who have not taken oral paliperidone, oral risperidone, or injectable risperidone, establish tolerability first.1
References: 1. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc. 2. Russu A, Kern Sliwa J, Ravenstijn P, et al. Maintenance dose conversion between oral risperidone and paliperidone palmitate 1 month: practical guidance based on pharmacokinetic simulations. Int J Clin Pract. 2018;72(6):e13089. 3. Samtani MN, Gopal S, Gassmann-Mayer C, et al. Dosing and switching strategies for paliperidone palmitate: based on population pharmacokinetic modelling and clinical trial data. CNS Drugs. 2011;25(10):829-845. 4. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.

