FOR US HEALTHCARE PROFESSIONALS ONLY
With INVEGA SUSTENNA®, you can be confident patients receive 1 month of medication1,2*
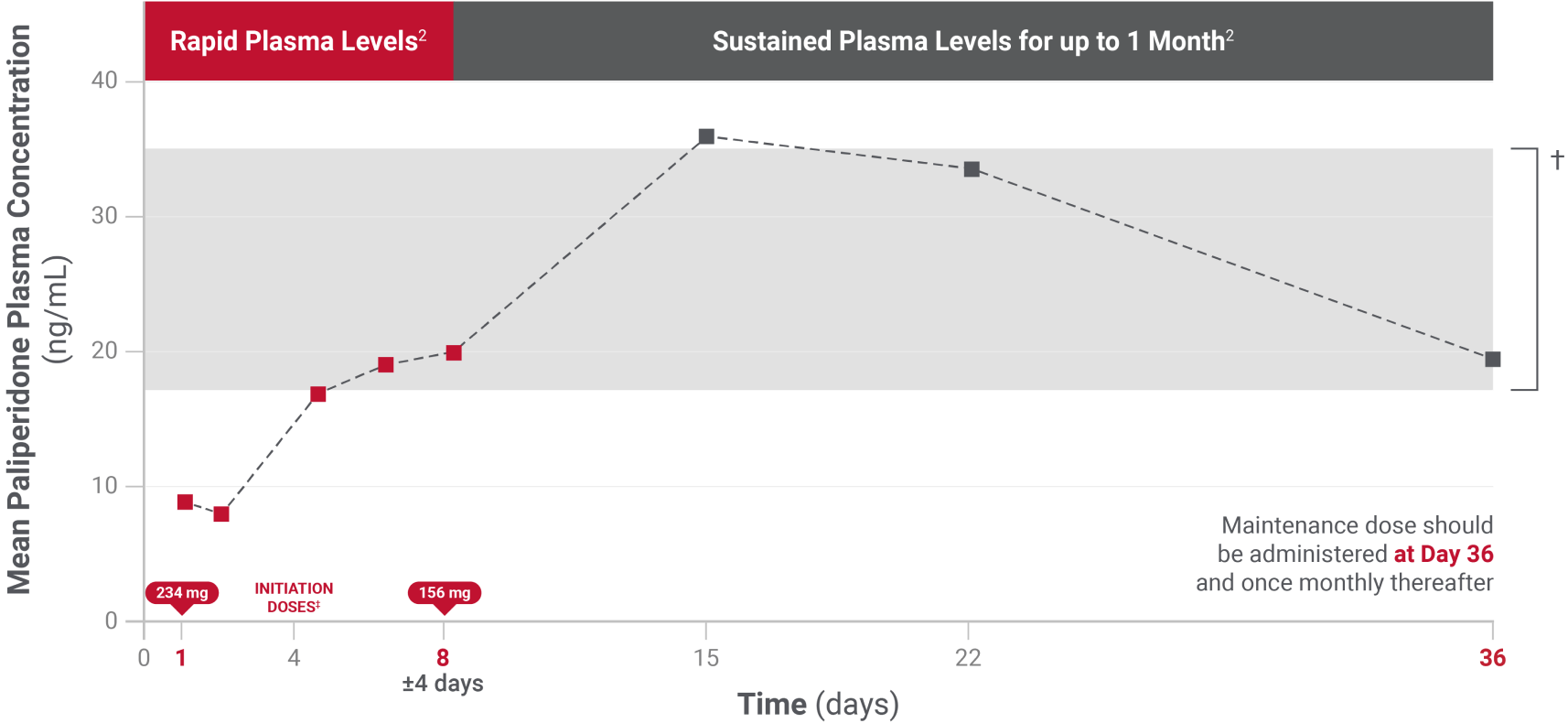
- No oral supplementation required during initiation, including Day 11
- Correlation to clinical effect has not been established
- Due to the difference in median pharmacokinetic profiles between INVEGA SUSTENNA® and oral INVEGA® (paliperidone) tablets, use caution when directly comparing their pharmacokinetic properties1
*After both initiation doses.1
†INVEGA SUSTENNA® initiation regimen allowed patients to stay in a median exposure window of 6-12 mg extended-release oral paliperidone.1
‡Initiation doses must be administered in the deltoid muscle.1
Build a plan that moves beyond the peaks and troughs of daily oral dosing with INVEGA SUSTENNA®3

Please see Important Safety Information and full Prescribing Information for RISPERDAL® (risperidone).
*The y-axis represents the typical paliperidone plasma concentration in nanograms per milliliter (ng/mL). Since efficacy and specific levels will vary from patient to patient, the concentration depicted is not intended to represent or imply therapeutic blood levels of paliperidone.
There are no systematically collected data to specifically address switching patients with schizophrenia from other antipsychotics to INVEGA SUSTENNA®, or concerning concomitant administration with other antipsychotics.
For patients who have never taken oral paliperidone or oral or injectable risperidone, tolerability should be established with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA SUSTENNA®.
Patients transitioning from oral antipsychotics must follow the recommended initiation dosing of 234 mg (day 1) and 156 mg (day 8), both administered in the deltoid muscle. Please see the full Prescribing Information for initiating patients with mild renal impairment.1
The initiation regimen (234 mg followed by 156 mg one week later, both in the deltoid muscle) rapidly attains steady-state paliperidone concentrations with no need for oral antipsychotic supplementation.1
The recommended maintenance dose of INVEGA SUSTENNA® for treatment of schizophrenia is 117 mg. Some patients may benefit from lower or higher maintenance doses within the additional available strengths (39 mg, 78 mg, 156 mg, and 234 mg).1
References: 1. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc. 2. Samtani MN, Gopal S, Gassmann-Mayer C, et al. Dosing and switching strategies for paliperidone palmitate. CNS Drugs. 2011;25(10):829-845. 3 Data on file. Janssen Pharmaceuticals, Inc.
Back to Top