FOR US HEALTHCARE PROFESSIONALS ONLY
The safety of INVEGA HAFYERA® was similar to that of INVEGA TRINZA®1
The safety profile of INVEGA HAFYERA® was similar to that seen with INVEGA TRINZA® in the double-blind phase of the study1
Safety and adverse reactions: noninferiority study
- In the double-blind phase, 1.3% of patients in the INVEGA HAFYERA® group and 0.4% of patients in the INVEGA TRINZA® group discontinued due to adverse events
- The most common adverse reactions (≥5%) in the INVEGA HAFYERA® group were upper respiratory tract infection (12%), injection-site reaction (11%), weight increased (9%), headache (7%), and extrapyramidal symptoms* (7%)
- The most common adverse reactions (≥5%) in the INVEGA TRINZA® group were upper respiratory tract infection (13%), weight increased (8%), injection-site reaction (5%), extrapyramidal symptoms* (5%), and headache (5%)
Treatment-emergent adverse reactions (≥2%) during noninferiority study
| Double-Blind | ||
|---|---|---|
| INVEGA TRINZA® (n=224) | INVEGA HAFYERA® (n=478) | |
| Upper respiratory tract infection | 13% | 12% |
| Injection-site reaction | 5% | 11% |
| Weight increased | 8% | 9% |
| Headache | 5% | 7% |
| Extrapyramidal symptoms* | 5% | 7% |
| Akathisia | 4% | 4% |
| Psychosis | 3% | 3% |
| Urinary tract infection | 1% | 3% |
| Back pain | 1% | 3% |
| Musculoskeletal pain | 1% | 3% |
| Anxiety | 0% | 3% |
| Insomnia | 2% | 3% |
| Diarrhea | 1% | 2% |
No New Safety Signals Emerged
The safety profile observed for INVEGA HAFYERA® was consistent with previous studies of INVEGA SUSTENNA® and INVEGA TRINZA®, with no new safety signals emerging.
An examination of population subgroups in the INVEGA HAFYERA® trial did not reveal any evidence of differences in safety on the basis of age, gender, or race alone.
Weight gain: Weight gain has been reported with the use of atypical antipsychotics. Clinical monitoring of weight is recommended.
Extrapyramidal symptoms include: blepharospasms, bradykinesia, drooling, dyskinesia, dystonia, hypokinesia, musculoskeletal stiffness, muscle rigidity, muscle spasms, oculogyric crisis, Parkinsonism, Parkinsonism rest tremor, reduced facial expression, tardive dyskinesia.
Safety and adverse events: open-label extension study3
The most common (≥5%) treatment-emergent adverse events were headache (13.5%), blood prolactin increased (10.7%), hyperprolactinemia (7.3%), diarrhea (6.2%), weight increased (5.1%), and nasopharyngitis (5.1%).*
No new safety signals were identified in the open-label extension study relative to the double-blind study3
Treatment-emergent adverse events (≥5%) during open-label extension3
| INVEGA HAFYERA® (n=178) | |
|---|---|
| Headache | 13.5% |
| Prolactin increased* | 10.7% |
| Hyperprolactinemia* | 7.3% |
| Diarrhea | 6.2% |
| Weight increased | 5.1% |
| Nasopharyngitis | 5.1% |
*In the open-label extension design, investigators were not blinded to prolactin laboratory results. Comparisons between double-blind studies and open-label extension studies should not be made.
Study notes3
- 111 subjects (62.4%) experienced at least 1 TEAE during the study
- Eight subjects (4.5%) experienced serious TEAEs
- Six subjects terminated early due to TEAEs, and the most frequent cause was worsening of schizophrenia (n=4)
Patient ratings of injection-site pain were mild1-3
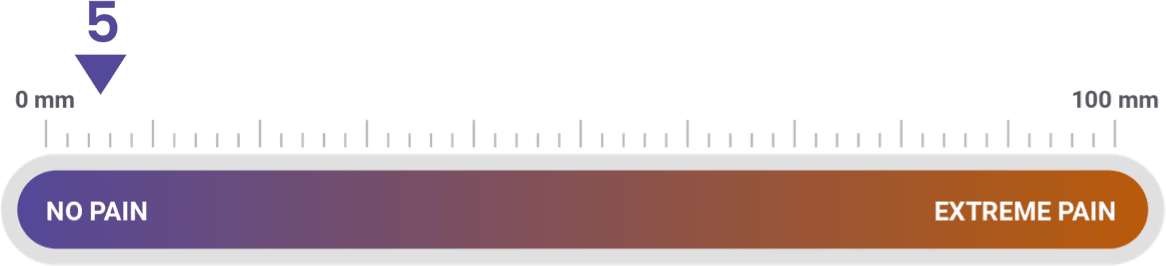
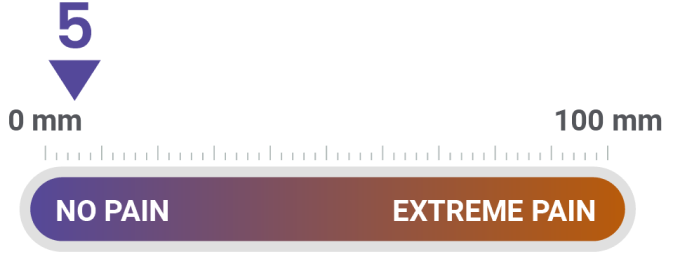
Patient ratings of injection-site pain were mild, according to Visual Analog Scale
No patients discontinued treatment because of injection-site pain.
30 minutes after injection, patients marked a Visual Analog Scale 100 millimeters in length, with 0 mm indicating no pain and 100 mm indicating extreme pain. Investigators measured each mark's distance from 0 mm to quantify injection-site pain.
The average score for the patient's evaluation of injection-site pain on a scale of 0 to 100 was approximately 16 at the open-label-phase endpoint and approximately 5 in both groups at the double-blind-phase endpoint.
Investigator ratings of injection site1
Induration, redness, and swelling were observed in 13% in the INVEGA HAFYERA® group and 9% in the INVEGA TRINZA® group during the double-blind phase.
References: 1. INVEGA HAFYERA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc. 2. Najarian D, Sanga P, Wang S, et al. A randomized, double-blind, multicenter, noninferiority study comparing paliperidone palmitate 6-month versus the 3-month long-acting injectable in patients with schizophrenia. Int J Neuropsychopharmacol. 2022;25(3):238-251. 3. Najarian D, Turkoz I, Knight RK, et al. Long-term efficacy and safety of paliperidone 6-month formulation: an open-label 2-year extension of a 1-year double-blind study in adult participants with schizophrenia. Int J Neuropsychopharmacol. 2023;26(8):537-544.
Back to Top